Currently, there are 10 DNA-based alterations, or biomarkers, for which we have targeted therapies in NSCLC. Rare mutations and fusions include BRAF V600E, HER2, MET, NRG1, NTRK, RET, and ROS1.
How are rare mutations and fusions identified?
Lung cancer is diagnosed using a variety of tests, including imaging, lab tests, biopsies, and biomarker testing. In the United States, a variety of tests can be used to make a diagnosis of NSCLC. Importantly, comprehensive biomarker testing can help determine if your lung cancer has an underlying biomarker or “driver mutation” that might make it respond to certain targeted treatments. It is important to know this information because it will help guide you and your doctor to the most appropriate treatments for your type of lung cancer. Tests to identify whether you have biomarkers may be performed on samples of your tissue or on blood samples (liquid biopsy).
Other tests used to identify biomarkers include FISH (fluorescence in situ hybridization), IHC (immunohistochemistry) or NGS (next generation sequencing). NGS falls under the category of “comprehensive biomarker testing.” While NGS is often thought of as DNA-based testing, there is growing evidence that RNA-based testing may help identify more rare fusions. Ask your doctor about comprehensive biomarker testing and whether additional testing (such as RNA-based testing) might be appropriate for you.
It should also be noted that comprehensive biomarker testing is not only recommended at the time of diagnosis. Biomarker testing may be appropriate:
- When the doctors suspect lung cancer and have recommended a biopsy
- When a patient is already diagnosed with lung cancer
- When a patient's lung cancer recurs (comes back) after treatment
This chart compares the occurrence of the many biomarkers in NSCLC:
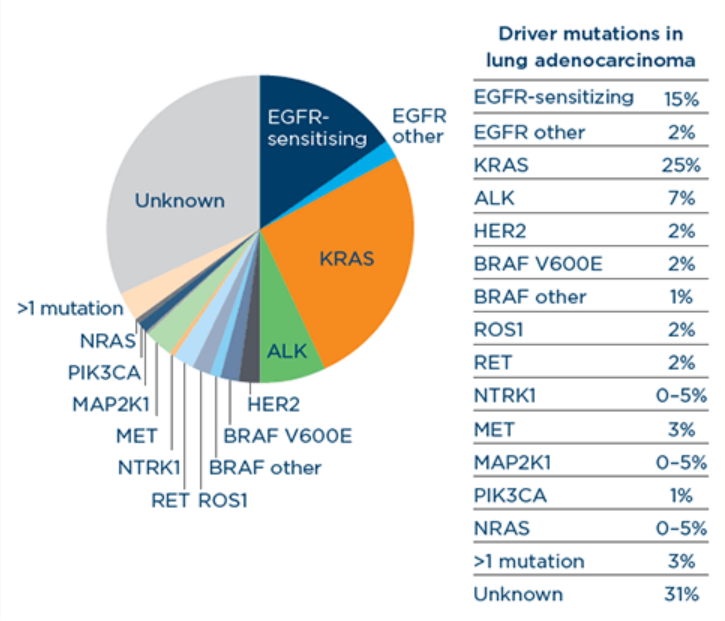
Many of the rarer mutations and fusions in NSCLC are known as “pan-cancer” alterations, meaning they may occur in several different types of cancer, not just lung cancer.
Treatment options
BRAF V600E
Mutations in the BRAF V600E gene occur in 1%-3% of lung adenocarcinoma patients. Most of these patients are current or former smokers.
There is currently one FDA-approved targeted treatment for patients with metastatic NSCLC with a BRAF V600E mutation, as detected by an FDA-approved test. This is a combination treatment of a BRAF kinase inhibitor, dabrafenib (Tafinlar®), with a MEK kinase inhibitor, trametinib (Mekinist®).
HER2
Mutations in the HER2 gene (also called ERBB2) are responsible for approximately 3% of nonsquamous NSCLCs. HER2 mutations are more commonly associated with younger women without a history of smoking.
There are currently three FDA-approved targeted drugs to treat NSCLC with HER2 mutations:
- Trastuzumab deruxtecan (Enhertu®): Approved for adult patients with unresectable or metastatic NSCLC whose tumors have activating HER2 (ERBB2) mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy.
- Zongertinib (Hernexeos®): Approved for treatment of adult patients with unresectable or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) tyrosine kinase domain activating mutations, as detected by an FDA-authorized test.
- Sevabertinib (Hyrnuo®): Approved for adult patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) whose tumors have HER2 (ERBB2) tyrosine kinase domain (TKD) activating mutations, as detected by an FDA-approved test, and who have received a prior systemic therapy.
MET
Disruptions of the MET (mesenchymal-epithelial transition) pathway can be caused by changes in the MET gene, including MET exon 14 skipping, and amplifications. Approximately 3%-4% of patients with NSCLC have a mutation that is caused by MET exon 14 skipping. Also, 25%-39% of nonsquamous NSCLC (without EGFR mutations) may produce high levels of the MET protein – termed MET overexpression. Patients with MET-positive lung cancers are most likely to have a smoking history; a minority are never-smokers.
There are currently two FDA-approved MET TKIs for exon 14 skipping mutations:
- Capmatinib (Tabrecta™): Approved for the treatment of adult patients with metastatic NSCLC whose tumors have a mutation that leads to MET exon 14 skipping, as detected by an FDA-approved test
- Tepotinib (Tepmetko®): Approved for the treatment of adult patients with metastatic NSCLC whose tumors have a mutation that leads to MET exon 14 skipping
There is currently one FDA-approved MET antibody-drug conjugate (ADC) for MET overexpression:
- Telisotuzumab vedotin-tllv (EmrelisTM): Approved under accelerated approval for the treatment of adult patients with locally advanced or metastatic non-squamous non-small cell lung cancer (NSCLC) with high c-Met protein overexpression (≥50% of tumor cells with strong [3+] staining), as determined by an FDA-approved test, who have received a prior systemic therapy
NRG1
The NRG1 gene encodes a protein (neuregulin-1) that plays a role in organ development. Fusions in NRG1 cause abnormal signaling that can lead to tumor growth. NRG1 fusions have been found in NSCLC, pancreatic cancer, and other cancer types. These fusions are rare, accounting for only about 0.2% of solid tumors, and current evidence points to poor outcomes with chemotherapy and immunotherapy.
There is currently one FDA-approved targeted drug to treat NSCLC caused by a NRG1 gene fusion:
- Zenocutuzumab-zbco (Bizengri®): Approved in adults with advanced, unresectable or metastatic non-small cell lung cancer (NSCLC) harboring a neuregulin 1 (NRG1) gene fusion with disease progression on or after prior systemic therapy. It is a bispecific antibody that binds HER2 and prevents binding of NRG1 to HER3. This disrupts the signaling pathway responsible for cell growth and proliferation.
NTRK
About 3%-4% of NSCLC patients have an NTRK (neurotrophic receptor kinase) gene fusion. NTRK fusions are more likely to be seen in patients who are light or never-smokers.
There are currently two FDA-approved NTRK TKIs:
- Entrectinib (Rozlytrek®): Approved for the treatment of adult and pediatric patients 12 years of age and older with solid tumors that:
- have an NTRK gene fusion with a known acquired resistance mutation,
- are metastatic or where surgical resection is likely to result in severe morbidity, and
- have progressed following treatment or have no satisfactory alternative treatment
- Larotrectinib (Vitrakvi®): Approved for the treatment of patients with NTRK solid tumors that:
- have an NTRK gene fusion without a known acquired resistance mutation
- are metastatic or where surgical resection is likely to result in severe morbidity, and
- have progressed following treatment or have no satisfactory alternative therapy
- Repotrectinib (AugtyroTM): Approved for the treatment of adult and pediatric patients 12 years of age and older with solid tumors that:
- have a neurotrophic tyrosine receptor kinase (NTRK) gene fusion, and
- are locally advanced or metastatic or where surgical resection is likely to result in severe morbidity
- have progressed following treatment or have no satisfactory alternative therapy
RET
Approximately 1% of NSCLC patients have a RET (rearranged during transfection) fusion. RET patients have been seen to have lung adenocarcinoma and be never-smokers.
There are currently two FDA-approved RET TKIs:
- Selpercatinib (Retevmo™): Approved for the treatment of adult patients with metastatic RET fusion-positive NSCLC, as detected by an FDA-approved test
- Pralsetinib (Gavreto™): Approved for the treatment of adult patients with metastatic RET fusion-positive NSCLC
ROS1
A ROS1 (receptor tyrosine kinase) rearrangement is a fusion between two genes, ROS1 and another gene. As with ALK, the fusion of the two genes produces an abnormal protein that causes cancer cells to grow and spread.
About 1%-2% of patients with NSCLC in the US and 2%-3% in East Asia have tumors with a ROS1 mutation. ROS1 tumors are more commonly found among younger patients (median age at diagnosis is 50 years), females, never-smokers, and patients with lung adenocarcinoma.
There are currently four FDA-approved ROS1 TKIs:
- Crizotinib (Xalkori®): Approved for patients with metastatic NSCLC whose tumors are ROS1-positive, as detected by an FDA-approved test
- Entrectinib (Rozlytrek®): Approved for adult patients with metastatic NSCLC whose tumors are ROS1-positive
- Repotrectinib (AugtyroTM): Approved for the treatment of adult patients with locally advanced or metastatic ROS1-positive NSCLC
- Taletrectinib (IbtroziTM): Approved for the treatment of adult patients with locally advanced or metastatic ROS1-positive NSCLC
Treatment side effects
BRAF V600E
Side effects of the BRAF V600E combination inhibitor vary by patient. Some common side effects of the BRAF V600E combination inhibitor include:
- Fatigue
- Nausea
- Vomiting
- Diarrhea
- Dry skin
- Decreased appetite
- Fever
- Swelling of the hands or feet
- Rash
- Bleeding
- Cough
- Difficulty breathing
- Chills
Among the more serious but less common side effects of the BRAF V600E combination inhibitor are:
- Vision toxicities
- Pneumonitis
- Cardiomyopathy
- Hyperglycemia
HER2
Side effects of trastuzumab deruxtecan vary by patient. Some common side effects of trastuzumab deruxtecan include:
- Nausea
- Fatigue
- Vomiting
- Alopecia
- Constipation
- Musculoskeletal pain
- Decreased appetite
- Diarrhea
- Anemia
Among the more serious but less common side effects of trastuzumab deruxtecan are:
- Interstitial lung disease/pneumonitis
- Neutropenia
- Left ventricular dysfunction
MET
Side effects of the MET TKIs vary by drug and by patient. Some common side effects of the MET TKIs as a group include:
- Swelling of the hands or feet
- Nausea
- Vomiting
- Fatigue and weakness
- Musculoskeletal pain
- Shortness of breath
- Loss of appetite
Among the more serious but less common side effects of the MET TKIs as a group are:
- Pneumonitis
- Liver damage
NRG1
Side effects of zenocutuzumab-zbco vary by patient. Some common side effects of zenocutuzumab-zbco include:
- Diarrhea
- Nausea
- Musculoskeletal pain
- Cough
- Dyspnea (trouble breathing)
- Fatigue
- Rash
- Infusion-related reactions
- Vomiting
- Abdominal pain
- Edema
Among the more serious but less common side effects of zenocutuzumab-zbco are:
- Pneumonia
- Acute kidney injury
- Dysphagia (trouble swallowing)
- Bradycardia
NTRK
Side effects of the NTRK TKIs vary by drug and by patient.27,28 Some common side effects of NTRK inhibitors as a group include:
- Fatigue
- Nausea
- Vomiting
- Dizziness
- Constipation
- Diarrhea
- Cough
- High AST (aspartate aminotransferase) levels, indicating liver issues
- High ALT (alanine aminotransferase) levels, indicating liver issues
Among the more serious but less common side effects of the NTRK TKIs as a group are:
- Congestive heart failure
- Skeletal fractures
- Central nervous system effects
- Vision disorders
ROS1
Side effects of ROS1 TKIs vary by drug and by patient. Some common side effects of ROS1 TKIs as a group include:
- Nausea
- Vomiting
- Diarrhea
- Constipation
- Fatigue
- Muscle aches
Among the more serious but less common side effects of the ROS1 TKIs are:
- Vision disorders
- Low testosterone
- Skeletal fractures
- Central nervous system effects
- Hepatotoxicity
- Interstitial lung disease/pneumonitis
- Congestive heart failure
Crizotinib is also used to treat ALK-positive NSCLC. In that setting, side effects have also been observed related to rarer eye and testosterone side effects.
Get tips on managing treatment-related side effects.
Treatment challenges
Unfortunately, many patients on targeted therapies eventually develop drug resistance, meaning the drug stops working and their cancer progresses. Many research efforts are underway to understand drug resistance and find ways to combat it. For example, researchers are looking at various drug combinations that may help delay or prevent resistance from happening.
Another challenge with rare mutations and fusions, like the other more common biomarkers, in NSCLC is that they don’t seem to respond to immunotherapy drugs. Researchers are actively exploring ways to open immunotherapy options to patients with biomarker-driven NSCLC.
Emerging targets
There are several emerging biomarkers that are being studied in the context of NSCLC. Therefore, it is so important that all patients with NSCLC get timely access to comprehensive biomarker testing. Even if a patient does not have a currently actionable biomarker, research is pushing forward to identify new targets and new treatments.
Questions to ask your doctor
- Did I receive comprehensive biomarker testing? If not, can I?
- Am I eligible for a clinical trial?
- Am I eligible for immunotherapy?
- What are my options if my tumor develops resistance to my treatment?

